Abstract
Introduction: Allogeneic hematopoietic cell transplantation (HCT) is the treatment of choice for a growing number of adults with acute leukemia (lymphoblastic [ALL], myeloid [AML]) or myelodysplastic syndrome (MDS). However, the potent anti-leukemic effect of HCT needs to be balanced by the high risk of non-relapse mortality (NRM) during the first year after HCT. Pre-HCT risk calculators such as the HCT comorbidity index (HCT-CI) facilitate stratification of NRM risk, but high inter-user variability and limited predictive power has limited the routine use of this tool in the clinical setting. Sarcopenia (loss of lean muscle mass) is an important cause of age-related functional decline in the general population, and is a predictor of NRM in patients with solid tumors, independent of age or pre-treatment comorbidity. The prognostic importance of sarcopenia on post-HCT outcomes has received limited attention.
Methods: Using a retrospective cohort design, 947 consecutive patients with acute leukemia or MDS, age ≥18y, who underwent a first HCT between 2007 to 2014 at City of Hope were included in the study. Skeletal muscle area was ascertained from pre-HCT abdominal computed tomography (CT) scans using image analysis software (SliceOmatic; Tomovision, Quebec, Canada). This report is limited to 868 (91.7%) patients with CT scans ≤150 days (d) prior to HCT (median: 28d [range: 0-150]). Measurements were made by trained researchers blinded to patient demographics and HCT outcome; 3rd lumbar vertebra was used as a landmark because of its high correlation with whole-body muscle mass (J Clin Oncol 2016 34:1339). Skeletal muscle index (SMI) was calculated as the ratio of skeletal muscle area (cm2) divided by height (m)2. Sex and body mass index-specific cutoff values of low SMI were used to identify patients with sarcopenia (J Clin Oncol 2013 31:1539). NRM at 1y was the primary endpoint, defined as time from HCT to death unrelated to relapse or disease progression; relapse/disease progression was considered a competing risk event. Length of hospitalization was the secondary endpoint, defined as time from HCT to hospital discharge; death prior to discharge was considered a competing risk event. Cumulative incidence of NRM was calculated taking into consideration competing risk. We used multivariable Fine and Gray regression analysis to calculate the hazard ratio (HR) estimates and 95% confidence intervals (CI), adjusted for relevant covariates (demographic, disease risk, donor source, conditioning intensity).
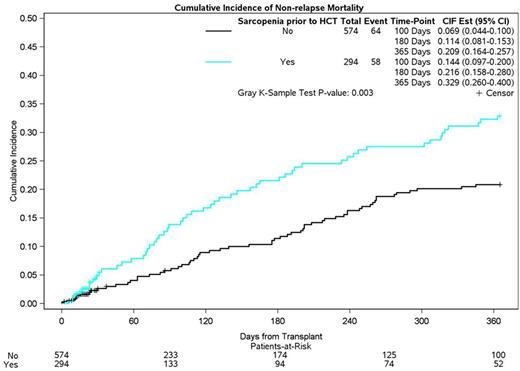
Results: Median age at HCT was 51y (range: 18-74); 54.1% were male; 52.0% were non-Hispanic white; Diagnoses: ALL (N=233 [26.9%]), AML (N=468 [53.9%]), MDS (N=167 [19.2%]); 52.5% had a high (≥3) HCT-CI score; Donor type: matched related (43.7%), matched unrelated (51.2%), other (5.2%); 50.7% underwent myeloablative conditioning; 43.4% developed grades 2-4 acute graft versus host disease; 294 (34%) had sarcopenia pre-HCT and 299 (26%) patients died within the first year of HCT - NRM (N=132), relapse-related (N=97). Adjusted cumulative incidence of NRM at 1-year was higher for sarcopenic compared to non-sarcopenic patients (32.9% vs. 20.9%, p=0.003; Figure). Sarcopenia was associated with a 1.7-fold (HR=1.68 [CI: 1.27-2.39]) risk of NRM at 1y when compared to those with normal muscle mass. Median length of hospitalization was also longer (30d vs. 28d; p<0.001) in sarcopenic patients. Sarcopenia and HCT-CI : The risk of NRM was highest among patients with sarcopenia and a high HCT-CI score (HR=2.05 [CI: 1.23-3.42]); a high HCT-CI score with normal muscle mass was not associated with excess NRM risk (HR=1.30 [CI: 0.79-2.16]; referent: low HCT-CI, normal muscle mass).
Conclusion: Sarcopenia is an important and independent predictor of NRM after HCT, with potential additional downstream impacts on health-economic outcomes such as length of hospitalization. Taken together, these data form the basis for real-time decision making prior to HCT (e.g. conditioning intensity, pre-habilitation), or during HCT (e.g. dietary optimization, increased supportive care services, resistance training), setting the stage for strategies to decrease the risk of treatment-related complications after HCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal